Bubble PPM for
Medtech & Medical Devices
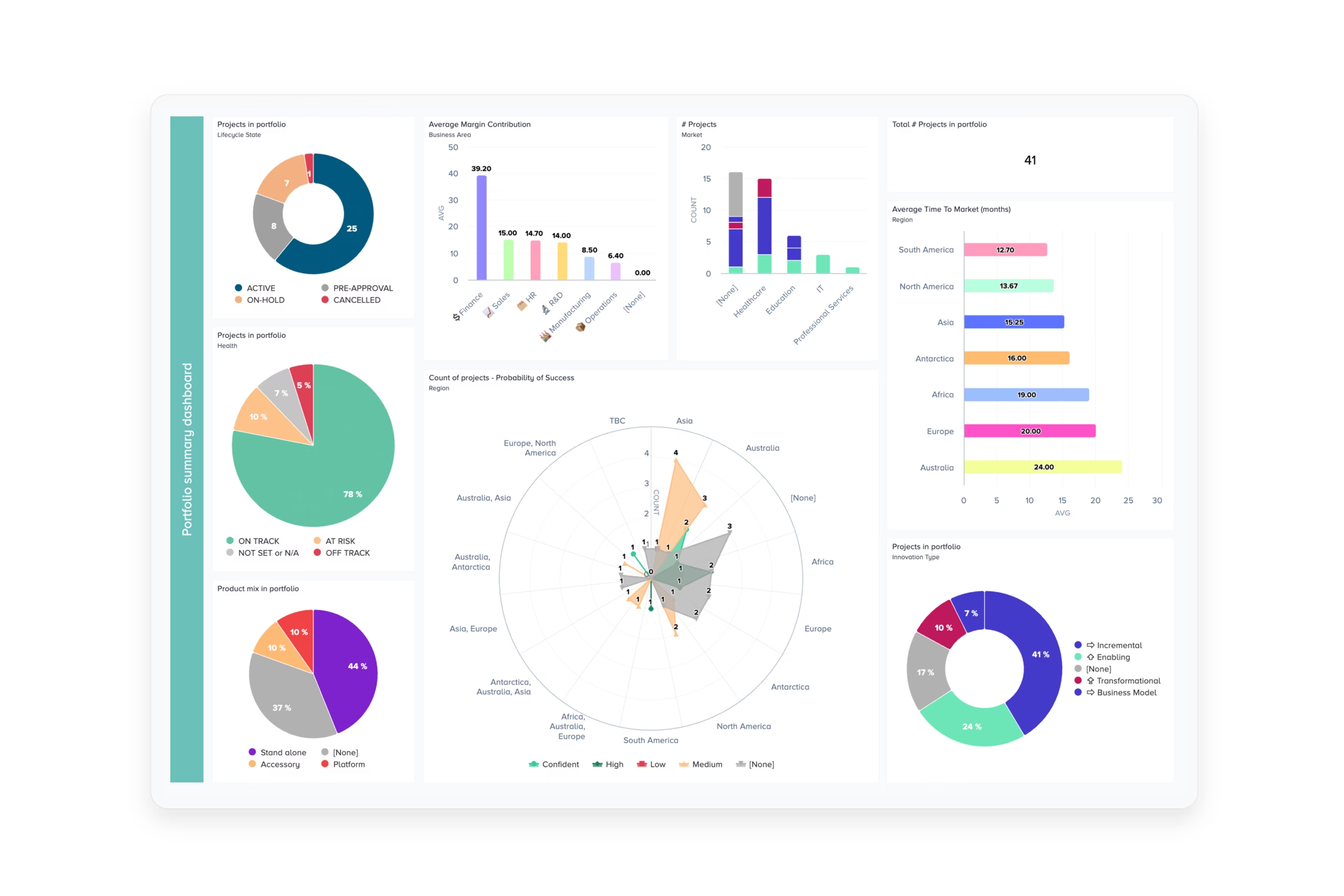
Realize project and pipeline value
Our PPM for MedTech sector solution helps medical device companies manage their technical innovations and improve the efficiency of their R&D efforts.
Driving innovation and compliance
Project and Portfolio Management helps MedTech Manufacturers by delivering strategic clarity from the boardroom to the lab floor.
For senior leaders, that often means accelerating time-to-market while managing risk. Delivery teams benefit from integrated tools that streamline project execution, track milestones, ensure compliance documentation, and enhance cross-functional collaboration.
Combined, a well designed PPM solution should help your organization stay competitive in an increasingly complex and regulated industry:
- Align R&D initiatives with regulatory and market demands
- Gain portfolio-level visibility to prioritize investments
- Balance resources across chartered and non-chartered projects
- Track value delivered from your core technologies / platforms
- Maintain quality over time, regardless of who’s delivering projects
- Innovate faster
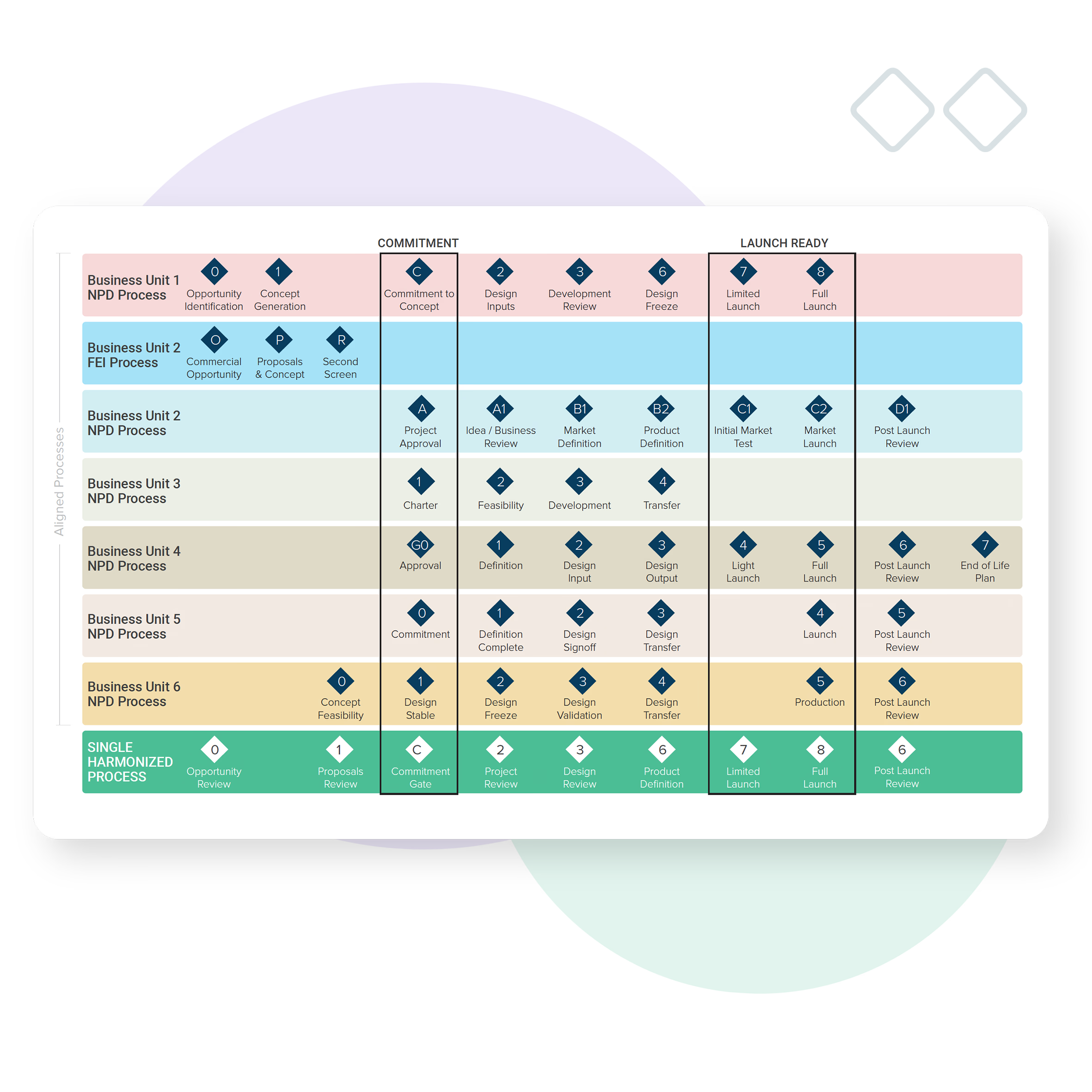
Research and technology program management
MedTech can require a special type of process management.
Research and Technology projects and programs may need to be connected, but managed separately, through their own development gates.
Bubble PPM’s configurable entity hierarchy ensures investments are optimized, benefits are tracked and dependencies are clear.
Link revenue from enabling research to future products
With over 25 years of portfolio & project management best practices built into the platform, our PPM for MedTech configuration helps senior leaders to prioritize and link the critical activities of all their teams.
With clear links to strategy, teams can organize a coherent approach to tracking research investments towards revenue from future products.
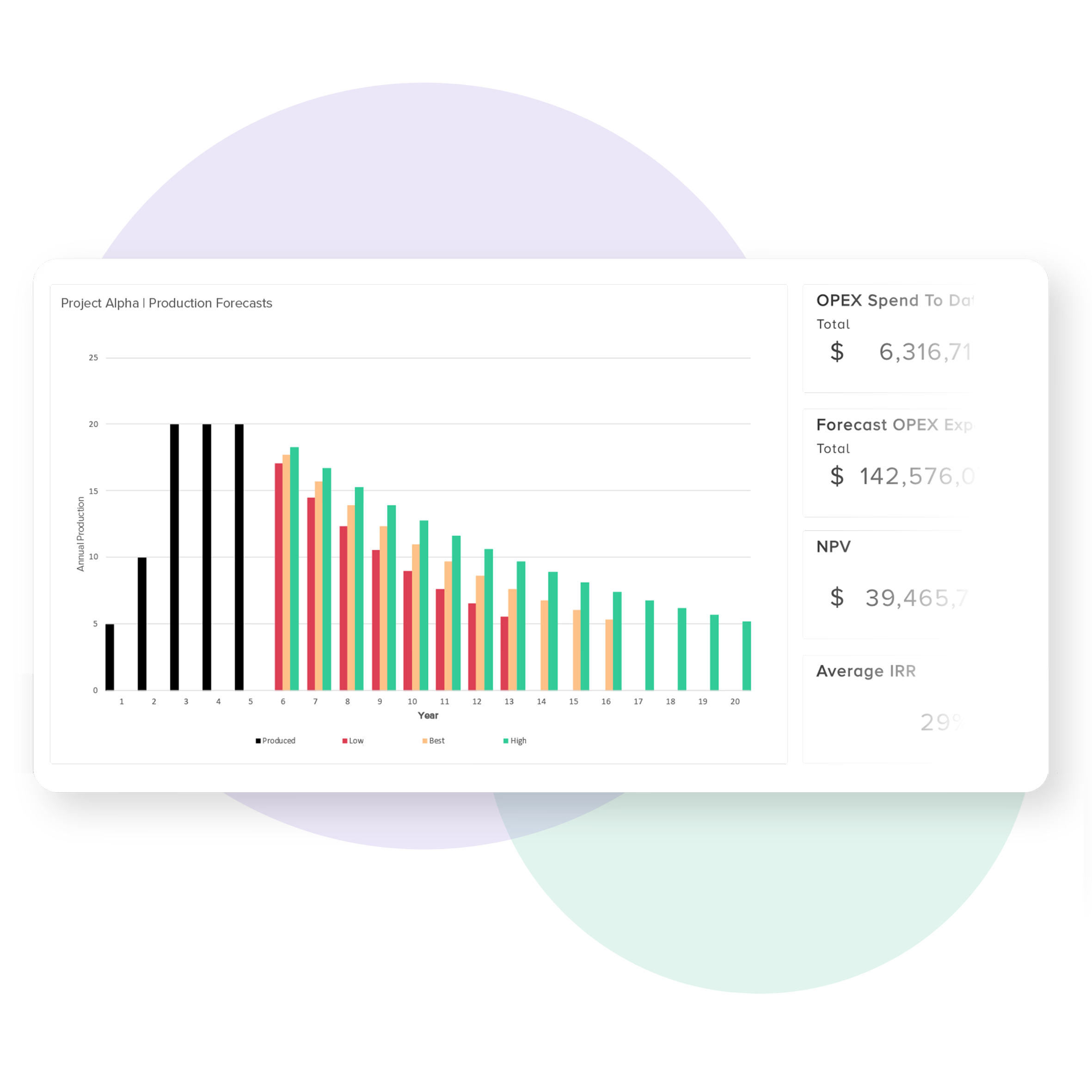
Improve the management of delivery forecasts
Bubble PPM facilitates the active management of priority actions, risk assessments, and potential project outcomes.
It enables project teams to highlight, and justify, the key areas where more data are required to mitigate uncertainty and provides senior management with insight into potential best and worse case scenarios.
Monitor risks throughout the project lifecycle
The majority of problems affecting Medical Devices get introduced during the early phases of development.
Create snapshotted ‘change record logs’ for things like Risks & Issues to help you adhere to requirements for quality systems such as Design Control and Design History File (DHF) reporting.
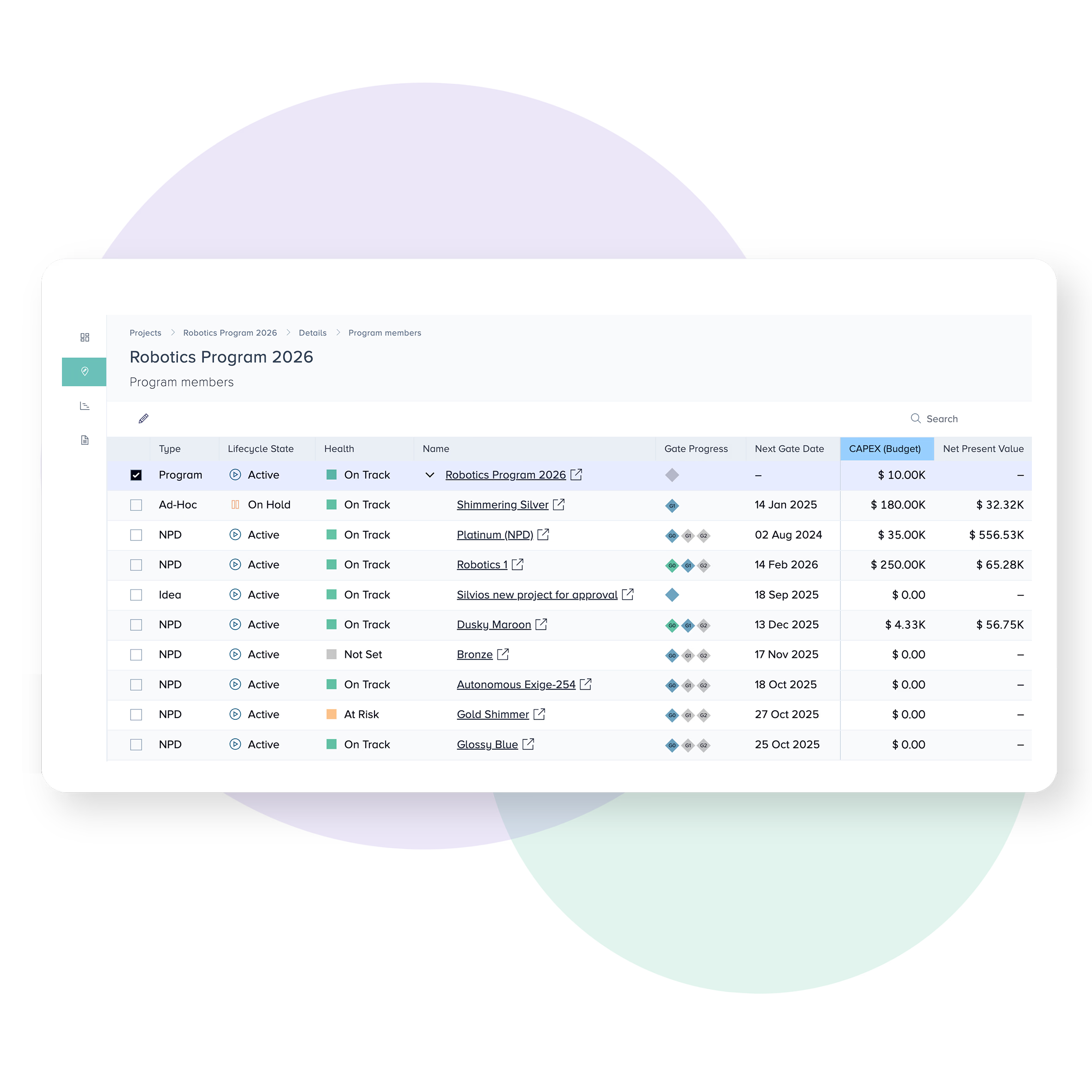
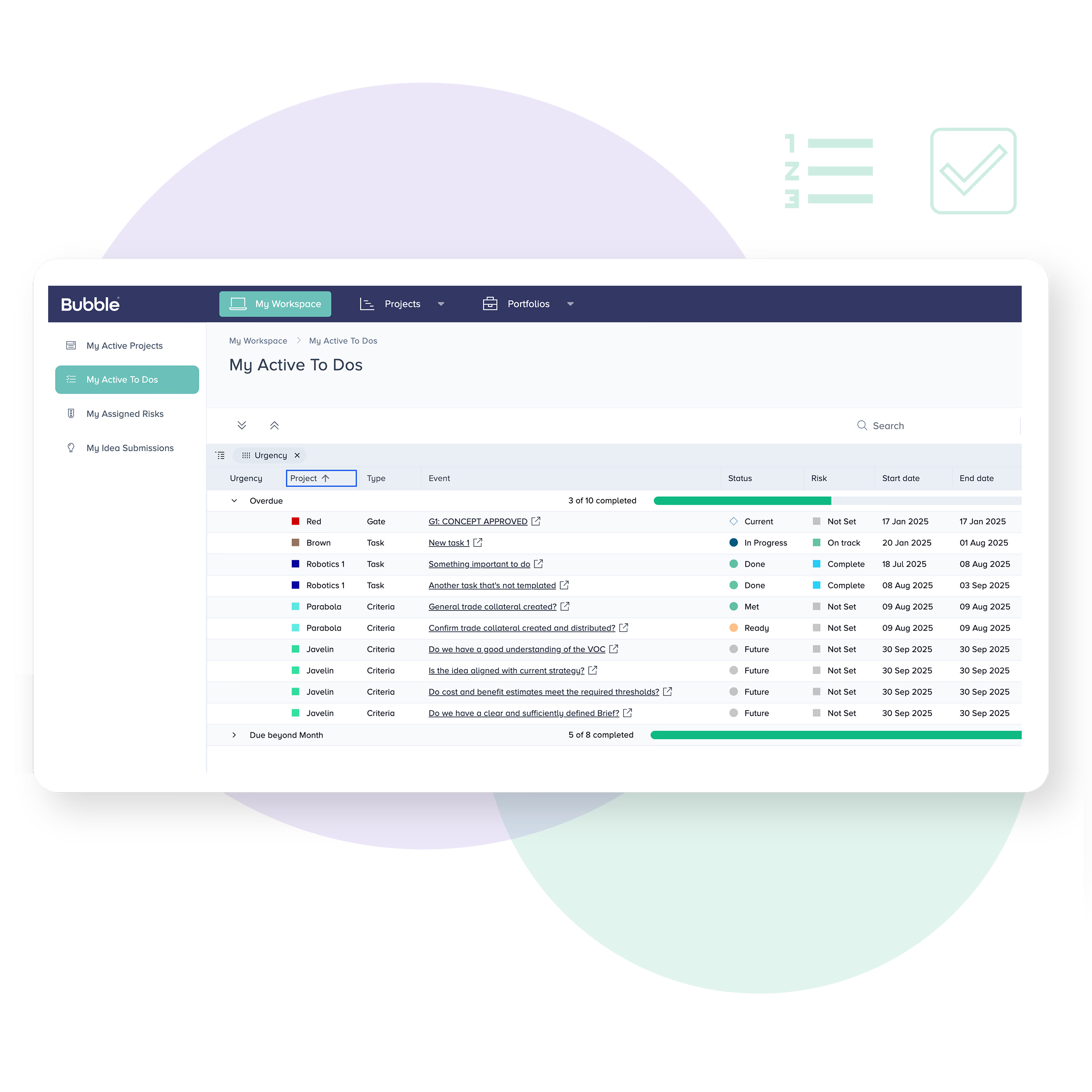
Task driven reporting
The complexity of MedTech and medical device development can make it hard to know who’s responsible for what. Bubble PPM allows each user to view their personal tasks and responsibilities according to the projects, departments or portfolios they work with.
Upcoming activities can be reviewed, shared or re-assigned with ease. Completed activities get rolled up to the system level for automated status reporting.
Harmonization of process
Bubble PPM ensures you’re delivering to a global view, and adherence to a common agreed workflow, by aligning the activity and management of divisions, programs and projects (regardless of type, size or phase of maturity).
Whether you’re delivering complex device portfolios that require a range of product solutions to suit different markets or growing through Mergers and Acquisitions (M&A) Bubble PPM can eliminate the pain of process harmonization across products, divisions or businesses to create one clear view.
Track product and supply chain insights
Managing supply chain risk helps device developers ensure their go to market strategy stays on track.
Bubble PPM allows you to incorporate supply chain insights, Stock Keeping Unit (SKU) trackers, and dashboards into the heart of your product development process.
Transform medical device development projects
Bubble PPM provides oversight of all your projects and deliverables regardless of complexity or volume.
Enhance risk management
Proactively identify, assess, and mitigate design and production risks through early issue resolution.
Streamline compliance
Work in accordance to FDA, ISO, and other standards to accelerate audits and approvals, and minimize time-to-market.
Optimize portfolio oversight
Gain real-time leadership visibility into all projects across R&D and manufacturing pipelines to deliver maximum strategic return.
Improve quality assurance
Ensure every device meets regulatory and customer quality expectations by embedding your quality checkpoints and workflows directly into project phases.
Data-driven decision gates
Improve gate reviews with live KPIs, business critical criteria’s and risk summaries to inform go/no-go decisions and reduce effort on low-value initiatives
Assess business cases
Standardize project evaluation criteria across projects, departments or business groups to improve funding allocation and alignment to corporate objectives.
It’s fully customizable to
your unique needs
Our out-of-the-box system is quickly configured.
Module based system
Each is easily adapted to deliver against challenges that apply to your organization.
It scales with ease as your business evolves
Our goal is to ensure customers have confidence in portfolio optimization and project execution. Our platform delivers:
Visibility
A single source-of-truth for all project data
Accountability
In-built project governance that follows your processes.
Efficiency
Automated dashboards and reports to save time.